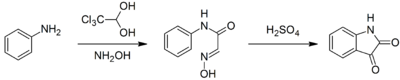Chloral, also known as trichloroacetaldehyde, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.
hloral can be produced by chlorination of ethanol, as reported in 1832 by Justus von Liebig.
hloral can be produced by chlorination of ethanol, as reported in 1832 by Justus von Liebig.
Aside from its tendency to hydrate, chloral is most notable as a building block in the synthesis of DDT. For this purpose, chloral is treated with chlorobenzene in the presence of a catalytic amount of sulfuric acid:
- Cl3CCHO + 2 C6H5Cl → Cl3CCH(C6H4Cl)2 + H2O
This reaction was described by Othmar Zeidler in 1874.
Chloral is also used to form chloroform by treating it with sodium hydroxide.
Chloral hydrate is a sedative and hypnotic drug as well as a chemical reagent and precursor. The name chloral hydrate indicates that it is formed from chloral (trichloroacetaldehyde) by the addition of one molecule of water. Its chemical formula is C2H3Cl3O2.
It was discovered through the chlorination of ethanol in 1832 by Justus von Liebig in Gießen. Its sedative properties were first published in 1869 and subsequently, because of its easy synthesis, its use was widespread. It was widely used recreationally and misprescribed in the late 19th century. Chloral hydrate is soluble in both water and alcohol, readily forming concentrated solutions. A solution of chloral hydrate in alcohol called "knockout drops" was used to prepare a Mickey Finn. More reputable uses of chloral hydrate include its use as a clearing agent of chitin (and fibers) and as a key ingredient of Hoyer's mounting medium, which is used for slide-mounted observation of organisms under the microscope.
It is, together with chloroform, a minor side-product of the chlorination of water when organic residues are present in the water, though concentrations rarely exceed 5 micrograms per litre (µg/L)
Chloral hydrate is produced from chlorine and ethanol in acidic solution. In basic conditions the haloform reaction takes place and chloroform is produced.
- 4 Cl2 + C2H5OH + H2O → Cl3CCH(OH)2 + 5 HCl
Building block
Chloral hydrate is a starting point for the synthesis of more complex chemicals. It is the starting material for the production of chloral, which is produced by the distillation of a mixture of chloral hydrate and sulfuric acid, which serves as the desiccant.Notably, it is used to synthesize isatin. In this synthesis, chloral hydrate reacts with aniline and hydroxylamine to give a condensation product which cyclicizes in sulfuric acid to give the target compound:Hypnotic
Chloral hydrate is used for the short-term treatment of insomnia and as a sedative before minor medical or dental treatment. It was largely displaced in the mid-20th century by barbiturates and subsequently by benzodiazepines. It was also formerly used in veterinary medicine as a general anesthetic. Today, it is commonly used as an ingredient in the veterinary anesthetic Equithesin. It is also still used as a sedative prior to EEG procedures, as it is one of the few available sedatives that does not suppress epileptiform discharges.In therapeutic doses for insomnia chloral hydrate is effective within 60 minutes, it is metabolized within four minutes into trichloroethanol by erythrocytes and plasma esterases and many hours later into trichloroacetic acid. Higher doses can depress respiration and blood pressure.Long-term use of chloral hydrate is associated with a rapid development of tolerance to its effects and possible addiction as well as adverse effects including rashes, gastric discomfort and severe renal, cardiac and hepatic failureAcute overdosage is often characterized by nausea, vomiting, confusion, convulsions, slow and irregular breathing, cardiac arrhythmia, and coma. The plasma, serum or blood concentrations of chloral hydrate and/or trichloroethanol, its major active metabolite, may be measured to confirm a diagnosis of poisoning in hospitalized patients or to aid in the medicolegal investigation of fatalities. Accidental overdosage of young children undergoing simple dental or surgical procedures has occurred. Hemodialysis has been used successfully to accelerate clearance of the drug in poisoning victims.Chloral hydrate exerts its pharmacological properties via enhancing the GABA receptor complex. It is moderately addictive, as chronic use is known to cause dependency and withdrawal symptoms. The chemical can potentiate various anticoagulants and is weakly mutagenic in vitro and in vivo.Chloral hydrate is now illegal in the United States without a prescription. Chloral hydrate is a schedule IV controlled substance in the United States. Its properties have sometimes led to its use as a date rape drug. Chloral hydrate is not a controlled substance in the United Kingdom.Chloral hydrate is also an ingredient used for Hoyer's solution, a mounting medium for microscopic observation of diverse organisms such as bryophytes, ferns, seeds, and small arthropods (especially mites). One recipe for making Hoyer's is dissolving gum arabic (30.0 g) in water (50.0 mL), then adding chloral hydrate (200.0 g), and then finally adding glycerol (16.0 mL). An advantage of this medium include an excellent refraction index and clearing (macerating) properties of the small specimens (especially advantageous if specimens require observation with differential interference contrast microscopy). The major disadvantage of Hoyer's is its susceptibility to the effects of dehydration, which causes the mountant to crystallize and threatening the slide to become unusable. It is therefore absolutely necessary, after drying a mounted specimen, to thoroughly ring (2 layers are best) cover slips with a protective coating (e.g., insulating Glyptol), which prevents rehydration and mountant deterioration. Chloral hydrate reportedly does not effectively clear larger specimens, or arthropods that are more heavily sclerotized (e.g., larger insects). These should first be cleared with another product (e.g., 10% KCl), and then mounted in Hoyer's. Other disadvantages of Hoyer's (principally due to chloral hydrate) include toxicity (see above), and procurement problems due to chloral hydrate being a controlled substance.