Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an indole ring are called indoles. The indolic amino acid tryptophan is the precursor of the neurotransmitter serotonin.

Properties
Indole is a solid at room temperature. Indole can be produced by bacteria as a degradation product of the amino acid tryptophan. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many flower scents (such as orange blossoms) and perfumes. It also occurs in coal tar.
The corresponding substituent is called indolyl.
Indole undergoes electrophilic substitution, mainly at position 3. Substituted indoles are structural elements of (and for some compounds the synthetic precursors for) the tryptophan-derived tryptamine alkaloids like the neurotransmitter serotonin, and melatonin. Other indolic compounds include the plant hormone Auxin (indolyl-3-acetic acid, IAA), the anti-inflammatory drug indomethacin, the betablocker pindolol, and the naturally occurring hallucinogen dimethyltryptamine (N,N-DMT).
The name indole is a portmanteau of the words indigo and oleum, since indole was first isolated by treatment of the indigo dye with oleum.
Preparation
Indole is a major constituent of coal-tar, and the 220–260 °C distillation fraction is the main industrial source of the material. Indole and its derivatives can also be synthesized by a variety of methods. The main industrial routes start from aniline.
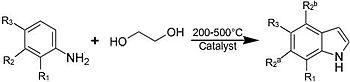
Illustrative of such large-scale syntheses, indole (and substituted derivatives) form via vapor-phase reaction of aniline with ethylene glycol in the presence of catalysts:
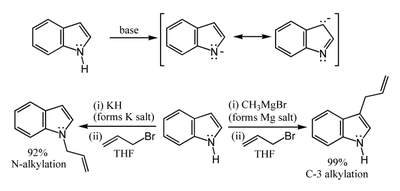 The N-H center has a pKa of 21 in DMSO, so that very strong bases such as sodium hydride or butyl lithium and water-free conditions are required for complete deprotonation. The resulting alkali metal derivatives can react in two ways. The more ionic salts such as the sodium or potassium compounds tend to react with electrophiles at nitrogen-1, whereas the more covalent magnesium compounds (indole Grignard reagents) and (especially) zinc complexes tend to react at carbon-3 (see figure below). In analogous fashion, polar aprotic solvents such as DMF and DMSO tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene favour C-3 attack.
The N-H center has a pKa of 21 in DMSO, so that very strong bases such as sodium hydride or butyl lithium and water-free conditions are required for complete deprotonation. The resulting alkali metal derivatives can react in two ways. The more ionic salts such as the sodium or potassium compounds tend to react with electrophiles at nitrogen-1, whereas the more covalent magnesium compounds (indole Grignard reagents) and (especially) zinc complexes tend to react at carbon-3 (see figure below). In analogous fashion, polar aprotic solvents such as DMF and DMSO tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene favour C-3 attack.
Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole.
 Due to the electron-rich nature of indole, it is easily oxidized. Simple oxidants such as N-bromosuccinimide will selectively oxidize indole 1 to oxindole (4 and 5).
Due to the electron-rich nature of indole, it is easily oxidized. Simple oxidants such as N-bromosuccinimide will selectively oxidize indole 1 to oxindole (4 and 5).

Properties
Indole is a solid at room temperature. Indole can be produced by bacteria as a degradation product of the amino acid tryptophan. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many flower scents (such as orange blossoms) and perfumes. It also occurs in coal tar.
The corresponding substituent is called indolyl.
Indole undergoes electrophilic substitution, mainly at position 3. Substituted indoles are structural elements of (and for some compounds the synthetic precursors for) the tryptophan-derived tryptamine alkaloids like the neurotransmitter serotonin, and melatonin. Other indolic compounds include the plant hormone Auxin (indolyl-3-acetic acid, IAA), the anti-inflammatory drug indomethacin, the betablocker pindolol, and the naturally occurring hallucinogen dimethyltryptamine (N,N-DMT).
The name indole is a portmanteau of the words indigo and oleum, since indole was first isolated by treatment of the indigo dye with oleum.
Preparation
Indole is a major constituent of coal-tar, and the 220–260 °C distillation fraction is the main industrial source of the material. Indole and its derivatives can also be synthesized by a variety of methods. The main industrial routes start from aniline.
Illustrative of such large-scale syntheses, indole (and substituted derivatives) form via vapor-phase reaction of aniline with ethylene glycol in the presence of catalysts:
Leimgruber-Batcho indole synthesis
 One of the oldest and most reliable methods for synthesizing substituted indoles is the Fischer indole synthesis, developed in 1883 by Emil Fischer. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions. Indole can still be synthesized, however, using the Fischer indole synthesis by reacting phenylhydrazine with pyruvic acid followed by decarboxylation of the formed indole-2-carboxylic acid. This has also been accomplished in a one-pot synthesis using microwave irradiation
One of the oldest and most reliable methods for synthesizing substituted indoles is the Fischer indole synthesis, developed in 1883 by Emil Fischer. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions. Indole can still be synthesized, however, using the Fischer indole synthesis by reacting phenylhydrazine with pyruvic acid followed by decarboxylation of the formed indole-2-carboxylic acid. This has also been accomplished in a one-pot synthesis using microwave irradiation
Basicity
 Unlike most amines, indole is not basic. The bonding situation is completely analogous to that in pyrrole. Very strong acids such as hydrochloric acid are required to protonate indole. The protonated form has an pKa of −3.6. The sensitivity of many indolic compounds (e.g., tryptamines) under acidic conditions is caused by this protonation.
Unlike most amines, indole is not basic. The bonding situation is completely analogous to that in pyrrole. Very strong acids such as hydrochloric acid are required to protonate indole. The protonated form has an pKa of −3.6. The sensitivity of many indolic compounds (e.g., tryptamines) under acidic conditions is caused by this protonation.
Electrophilic substitution
The most reactive position on indole for electrophilic aromatic substitution is C-3, which is 1013 times more reactive than benzene. For example, Vilsmeier-Haack formylation of indole will take place at room temperature exclusively at C-3. Since the pyrrollic ring is the most reactive portion of indole, electrophilic substitution of the carbocyclic (benzene) ring can take place only after N-1, C-2, and C-3 are substituted.Nitrogen-H acidity and organometallic indole anion complexes
 The N-H center has a pKa of 21 in DMSO, so that very strong bases such as sodium hydride or butyl lithium and water-free conditions are required for complete deprotonation. The resulting alkali metal derivatives can react in two ways. The more ionic salts such as the sodium or potassium compounds tend to react with electrophiles at nitrogen-1, whereas the more covalent magnesium compounds (indole Grignard reagents) and (especially) zinc complexes tend to react at carbon-3 (see figure below). In analogous fashion, polar aprotic solvents such as DMF and DMSO tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene favour C-3 attack.
The N-H center has a pKa of 21 in DMSO, so that very strong bases such as sodium hydride or butyl lithium and water-free conditions are required for complete deprotonation. The resulting alkali metal derivatives can react in two ways. The more ionic salts such as the sodium or potassium compounds tend to react with electrophiles at nitrogen-1, whereas the more covalent magnesium compounds (indole Grignard reagents) and (especially) zinc complexes tend to react at carbon-3 (see figure below). In analogous fashion, polar aprotic solvents such as DMF and DMSO tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene favour C-3 attack.Carbon acidity and C-2 lithiation
After the N-H proton, the hydrogen at C-2 is the next most acidic proton on indole. Reaction of N-protected indoles with butyl lithium or lithium diisopropylamide results in lithiation exclusively at the C-2 position. This strong nucleophile can then be used as such with other electrophiles.Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole.
Oxidation
 Due to the electron-rich nature of indole, it is easily oxidized. Simple oxidants such as N-bromosuccinimide will selectively oxidize indole 1 to oxindole (4 and 5).
Due to the electron-rich nature of indole, it is easily oxidized. Simple oxidants such as N-bromosuccinimide will selectively oxidize indole 1 to oxindole (4 and 5).