 We know about the new popular form of Carbon- Fullerene (buckyball). There are now thirty or more forms of fullerenes, and also an extended family of linear molecules, carbon nanotubes. C60 is the first spherical carbon molecule, with carbon atoms arranged in a soccer ball shape. In the structure there are 60 carbon atoms and a number of five-membered rings isolated by six-membered rings. The second, slightly elongated, spherical carbon molecule in the same group resembles a rugby ball, has seventy carbon atoms and is known as C70. C70’s structure has extra six-membered carbon rings, but there are also a large number of other potential structures containing the same number of carbon atoms. Their particular shapes depend on whether five-membered rings are isolated or not, or whether seven-membered rings are present. Many other forms of fullerenes up to and beyond C120 have been characterized, and it is possible to make other fullerene structures with five-membered rings in different positions and sometimes adjoining one another.
We know about the new popular form of Carbon- Fullerene (buckyball). There are now thirty or more forms of fullerenes, and also an extended family of linear molecules, carbon nanotubes. C60 is the first spherical carbon molecule, with carbon atoms arranged in a soccer ball shape. In the structure there are 60 carbon atoms and a number of five-membered rings isolated by six-membered rings. The second, slightly elongated, spherical carbon molecule in the same group resembles a rugby ball, has seventy carbon atoms and is known as C70. C70’s structure has extra six-membered carbon rings, but there are also a large number of other potential structures containing the same number of carbon atoms. Their particular shapes depend on whether five-membered rings are isolated or not, or whether seven-membered rings are present. Many other forms of fullerenes up to and beyond C120 have been characterized, and it is possible to make other fullerene structures with five-membered rings in different positions and sometimes adjoining one another. The important fact for nanotechnology is that useful dopant atoms can be placed inside the hollow fullerene ball. Atoms contained within the fullerene are said to be endohedral. Of course they can also be bonded to fullerenes outside the ball as salts, if the fullerene can gain electrons.
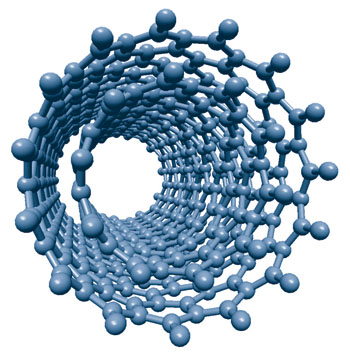 Endohedral fullerenes can be produced in which metal atoms are captured within the fullerene cages. Theory shows that the maximum electrical conductivity is to be expected for endohedral metal atoms, which will transfer three electrons to the fullerene. Fullerenes can be dispersed on the surface as a monolayer. That is, there is only one layer of molecules, and they are said to be mono dispersed. Provided fullerenes can be placed in very specific locations, they may be aligned to form a fullerene wire. Systems with appropriate material inside the fullerene ball are conducting and are of particular interest because they can be deposited to produce bead-like conducting circuits. Combining endohedrally doped structures with non-doped structures changes the actual composition of a fullerene wire, so that it may be tailored in-situ during patterning. Hence within a single wire, insulating and conducting regions may be precisely defined. One-dimensional junction engineering becomes realistic with fullerenes.
Endohedral fullerenes can be produced in which metal atoms are captured within the fullerene cages. Theory shows that the maximum electrical conductivity is to be expected for endohedral metal atoms, which will transfer three electrons to the fullerene. Fullerenes can be dispersed on the surface as a monolayer. That is, there is only one layer of molecules, and they are said to be mono dispersed. Provided fullerenes can be placed in very specific locations, they may be aligned to form a fullerene wire. Systems with appropriate material inside the fullerene ball are conducting and are of particular interest because they can be deposited to produce bead-like conducting circuits. Combining endohedrally doped structures with non-doped structures changes the actual composition of a fullerene wire, so that it may be tailored in-situ during patterning. Hence within a single wire, insulating and conducting regions may be precisely defined. One-dimensional junction engineering becomes realistic with fullerenes.Possibly more important than fullerenes are Carbon nanotubes, which are related to graphite. The molecular structure of graphite resembles stacked, one-atom-thick sheets of chicken wire - a planar network of interconnected hexagonal rings of carbon atoms. In conventional graphite, the sheets of carbon are stacked on top of one another, allowing them to easily slide over each other. That is why graphite is not hard, but it feels greasy, and can be used as a lubricant. When graphene sheets are rolled into a cylinder and their edges joined, they form CNTs. Only the tangents of the graphitic planes come into contact with each other, and hence their properties are more like those of a molecule.
CNTs come in a variety of diameters, lengths, and functional group content. CNTs today are available for industrial applications in bulk quantities up metric ton quantities from Cheap Tubes. Several CNT manufacturers have >100 ton per year production capacity for multi walled nanotubes.
A nanotube may consist of one tube of graphite, a one-atom thick single-wall nanotube, or a number of concentric tubes called multiwalled nanotubes. When viewed with a transmission electron microscope these tubes appear as planes. Whereas single walled nanotubes appear as two planes, in multi walled nanotubes more than two planes are observed, and can be seen as a series of parallel lines. There are different types of CNTs, because the graphitic sheets can be rolled in different ways. The three types of CNTs are Zigzag, Armchair, and Chiral. It is possible to recognize zigzag, armchair, and chiral CNTs just by following the pattern across the diameter of the tubes, and analyzing their cross-sectional structure.
Multi walled nanotubes can come in an even more complex array of forms, because each concentric single-walled nanotube can have different structures, and hence there are a variety of sequential arrangements. The simplest sequence is when concentric layers are identical but different in diameter. However, mixed variants are possible, consisting of two or more types of concentric CNTs arranged in different orders. These can have either regular layering or random layering. The structure of the nanotube influences its properties - including electrical and thermal conductivity, density, and lattice structure. Both type and diameter are important. The wider the diameter of the nanotube, the more it behaves like graphite. The narrower the diameter of the nanotube, the more its intrinsic properties depends upon its specific type.
Production
Arc Method Production
The carbon arc discharge method, initially used for producing C60 fullerenes, is the most common and perhaps easiest way to produce CNTs, as it is rather simple. However, it is a technique that produces a complex mixture of components, and requires further purification - to separate the CNTs from the soot and the residual catalytic metals present in the crude product. This method creates CNTs through arc-vaporization of two carbon rods placed end to end, separated by approximately 1mm, in an enclosure that is usually filled with inert gas at low pressure. Recent investigations have shown that it is also possible to create CNTs with the arc method in liquid nitrogen. A direct current of 50 to 100 A, driven by a potential difference of approximately 20 V, creates a high temperature discharge between the two electrodes. The discharge vaporizes the surface of one of the carbon electrodes, and forms a small rod-shaped deposit on the other electrode. Producing CNTs in high yield depends on the uniformity of the plasma arc, and the temperature of the deposit forming on the carbon electrode.
Production By Chemical Vapor Deposition
Chemical vapor deposition of hydrocarbons over a metal catalyst is a classical method that has been used to produce various carbon materials such as carbon fibers and filaments for over twenty years. Large amounts of CNTs can be formed by catalytic CVD of acetylene over cobalt and iron catalysts supported on silica or zeolite. The carbon deposition activity seems to relate to the cobalt content of the catalyst, whereas the CNTs’ selectivity seems to be a function of the pH in catalyst preparation. Fullerenes and bundles of single walled nanotubes were also found among the multi walled nanotubes produced on the carbon/zeolite catalyst. Some researchers are experimenting with the formation of CNTs from ethylene. Supported catalysts such as iron, cobalt, and nickel, containing either a single metal or a mixture of metals, seem to induce the growth of isolated single walled nanotubes or single walled nanotubes bundles in the ethylene atmosphere. The production of single walled nanotubes, as well as double-walled CNTs, on molybdenum and molybdenum-iron alloy catalysts has also been demonstrated. CVD of carbon within the pores of a thin alumina template with or without a Nickel catalyst has been achieved. Ethylene was used with reaction temperatures of 545°C for Nickel-catalyzed CVD, and 900°C for an uncatalyzed process. The resultant carbon nanostructures have open ends, with no caps. Methane has also been used as a carbon source. In particular it has been used to obtain ‘nanotube chips’ containing isolated single walled nanotubes at controlled locations. High yields of single walled nanotubes have been obtained by catalytic decomposition of an H2/CH4 mixture over well-dispersed metal particles such as Cobalt, Nickel, and Iron on magnesium oxide at 1000°C. It has been reported that the synthesis of composite powders containing well-dispersed CNTs can be achieved by selective reduction in an H2/CH4 atmosphere of oxide solid solutions between a non-reducible oxide such as Al2O3 or MgAl2O4 and one or more transition metal oxides. The reduction produces very small transition metal particles at a temperature of usually >800°C. The decomposition of CH4 over the freshly formed nanoparticles prevents their further growth, and thus results in a very high proportion of single walled nanotubes and fewer multi walled nanotubes.
Purification Methods
Purification of CNTs generally refers to the separation of CNTs from other entities, such as carbon nanoparticles, amorphous carbon, residual catalyst, and other unwanted species. The classic chemical techniques for purification have been tried, but they have not been found to be effective in removing the undesirable impurities. Three basic methods have been used with varying degrees of success, namely gas-phase, liquid-phase, and intercalation methods.
Generally, a centrifugal separation is necessary to concentrate the single walled nanotubes in low-yield soot before the micro filtration operation, since the nanoparticles easily contaminate membrane filters. The advantage of this method is that unwanted nanoparticles and amorphous carbon are removed simultaneously and the CNTs are not chemically modified. However 2-3 mol nitric acid is useful for chemically removing impurities.
It is now possible to cut CNTs into smaller segments, by extended sonication in concentrated acid mixtures. The resulting CNTs form a colloidal suspension in solvents. They can be deposited on substrates, or further manipulated in solution, and can have many different functional groups attached to the ends and sides of the CNTs.
In three Phases
1.Gas Phase
3.Intercalation
The first successful technique for purification of nanotubes was developed by Thomas Ebbesen and coworkers. Following the demonstration that nanotubes could be selectively attached by oxidizing gases these workers realized that nanoparticles, with their defect rich structures might be oxidised more readily than the relatively perfect nanotubes. They found that a significant relative enrichment of nanotubes could be achieved this way, but only at the expense of losing the majority of the original sample.
A new gas-phase method has been developed at the NASA Glenn Research Center to purify gram-scale quantities of single-wall CNTs. This method, a modification of a gas-phase purification technique previously reported by Smalley and others, uses a combination of high-temperature oxidations and repeated extractions with nitric and hydrochloric acid. This improved procedure significantly reduces the amount of impurities such as residual catalyst, and non-nanotube forms of carbon) within the CNTs, increasing their stability significantly.
2.Liquid Phase
The current liquid-phase purification procedure follows certain essential steps:
preliminary filtration- to get rid of large graphite particles;
dissolution- to remove fullerenes (in organic solvents) and catalyst particles (in concentrated acids)
centrifugal separation-
microfiltration- and
chromatography
It is important to keep the CNTs well-separated in solution, so the CNTs are typically dispersed using a surfactant prior to the last stage of separation.
3.Intercalation
An alternative approach to purifying multi walled nanotubes was introduced in 1994 by a Japanese research group. This technique made use of the fact that nanoparticles and other graphitic contaminants have relatively “open” structures and can therefore be more readily intercalated with a variety of materials that can close nanotubes. By intercalating with copper chloride, and then reducing this to metallic copper, the research group was able to preferentially oxidize the nanoparticles away, using copper as an oxidation catalyst. Since 1994, this has become a popular method for purification of nanotubes. “The first stage is to immerse the crude cathodic deposit in a molten copper chloride and potassium chloride mixture at 400°C and leave it for one week. The product of this treatment, which contains intercalated nanoparticles and graphitic fragments, is then washed in ion exchanged water to remove excess copper chloride and potassium chloride. In order to reduce the intercalated copper chloride-potassium chloride metal, the washed product is slowly heated to 500°C in a mixture of Helium and hydrogen and held at this temperature for 1 hour. Finally, the material is oxidized in flowing air at a rate of 10°C/min to a temperature of 555°C. Samples of cathodic soot which have been treated this way consist almost entirely of nanotubes. A disadvantage of this method is that some amount of nanotubes are inevitably lost in the oxidation stage, and the final material may be contaminated with residues of intercalates. A similar purification technique, which involves intercalation with bromine followed by oxidation, has also been described.
Dispersion
In some applications, achieving a stable dispersion can require other agents in the solution to prevent the CNTs from falling out of solution over time. Emulsifier T-60 (also known as Tween 60) is commonly used with Di water or Isopropyl Alcohol. Organic titanates can be used with Acetone and Xylene. The specific application determines whether these agents remain in the solution when further processing, or if they need to be removed. Some organic titanates can be removed by heating the solution above 250°C. The addition of the OH and COOH functional groups assists the CNTs dispersing in DI water and other solvents as well as the chemical bonding to other materials during further processing.
Functionalization
Pristine nanotubes are unfortunately insoluble in many liquids such as water, polymer resins, and most solvents. Thus they are difficult to evenly disperse in a liquid matrix such as epoxies and other polymers. This complicates efforts to utilize the nanotubes’ outstanding physical properties in the manufacture of composite materials, as well as in other practical applications which require preparation of uniform mixtures of CNTs with many different organic, inorganic, and polymeric materials.
To make nanotubes more easily dispersible in liquids, it is necessary to physically or chemically attach certain molecules, or functional groups, to their smooth sidewalls without significantly changing the nanotubes’ desirable properties. This process is called functionalization. The production of robust composite materials requires strong covalent chemical bonding between the filler particles and the polymer matrix, rather than the much weaker van der Waals physical bonds which occur if the CNTs are not properly functionalized.
Functionalization methods such as chopping, oxidation, and “wrapping” of the CNTs in certain polymers can create more active bonding sites on the surface of the nanotubes. For biological uses, CNTs can be functionalized by attaching biological molecules, such as lipids, proteins, biotins, etc. to them. Then they can usefully mimic certain biological functions, such as protein adsorption, and bind to DNA and drug molecules. This would enable medially and commercially significant applications such as gene therapy and drug delivery. In biochemical and chemical applications such as the development of very specific biosensors, molecules such as carboxylic acid (COOH), poly m-aminobenzoic sulfonic acid (PABS), polyimide, and polyvinyl alcohol (PVA) have been used to functionalize CNTs, as have amino acid derivatives, halogens, and compounds. Some types of functionalized CNTs are soluble in water and other highly polar, aqueous solvents.
Conductivity
Carbon nanotubules can be highly conducting, and hence can be said to be metallic. Their conductivity has been shown to be a function of their chirality, the degree of twist as well as their diameter. CNTs can be either metallic or semi-conducting in their electrical behavior. Conductivity in MWNTs is quite complex. Some types of “armchair”-structured CNTs appear to conduct better than other metallic CNTs. Furthermore, interwall reactions within multi walled nanotubes have been found to redistribute the current over individual tubes non-uniformly. However, there is no change in current across different parts of metallic single-walled nanotubes. The behavior of the ropes of semi-conducting single walled nanotubes is different, in that the transport current changes abruptly at various positions on the CNTs.
The conductivity and resistivity of ropes of single walled nanotubes has been measured by placing electrodes at different parts of the CNTs. The resistivity of the single walled nanotubes ropes was of the order of 10–4 ohm-cm at 27°C. This means that single walled nanotube ropes are the most conductive carbon fibers known. The current density that was possible to achieve was 10-7 A/cm2, however in theory the single walled nanotube ropes should be able to sustain much higher stable current densities, as high as 10-13 A/cm2. It has been reported that individual single walled nanotubes may contain defects. Fortuitously, these defects allow the single walled nanotubes to act as transistors. Likewise, joining CNTs together may form transistor-like devices. A nanotube with a natural junction (where a straight metallic section is joined to a chiral semiconducting section) behaves as a rectifying diode – that is, a half-transistor in a single molecule. It has also recently been reported that single walled nanotubes can route electrical signals at speeds up to 10 GHz when used as interconnects on semi-conducting devices.
Strength and Elasticity
The carbon atoms of a single sheet of graphite form a planar honeycomb lattice, in which each atom is connected via a strong chemical bond to three neighboring atoms. Because of these strong bonds, the basal plane elastic modulus of graphite is one of the largest of any known material. For this reason, CNTs are expected to be the ultimate high-strength fibers. Single walled nanotubes are stiffer than steel, and are very resistant to damage from physical forces. Pressing on the tip of a nanotube will cause it to bend, but without damage to the tip. When the force is removed, the nanotube returns to its original state. This property makes CNTs very useful as probe tips for very high-resolution scanning probe microscopy. Quantifying these effects has been rather difficult, and an exact numerical value has not been agreed upon.
Using atomic force microscopy, the unanchored ends of a freestanding nanotube can be pushed out of their equilibrium position, and the force required to push the nanotube can be measured. The current Young’s modulus value of single walled nanotubes is about 1 TeraPascal, but this value has been widely disputed, and a value as high as 1.8 Tpa has been reported. Other values significantly higher than that have also been reported. The differences probably arise through different experimental measurement techniques. Others have shown theoretically that the Young’s modulus depends on the size and chirality of the single walled nanotubes, ranging from 1.22 Tpa to 1.26 Tpa. They have calculated a value of 1.09 Tpa for a generic nanotube. However, when working with different multi walled nanotubes, others have noted that the modulus measurements of multi walled nanotubes using AFM techniques do not strongly depend on the diameter. Instead, they argue that the modulus of the multi walled nanotubes correlates to the amount of disorder in the nanotube walls. Not surprisingly, when multi walled nanotubes break, the outermost layers break first.
Thermal Conductivity and Expansion
CNTs have been shown to exhibit superconductivity below 20°K (aaprox. -253°C). Research suggests that these exotic strands, already heralded for their unparalleled strength and unique ability to adopt the electrical properties of either semiconductors or perfect metals, may someday also find applications as miniature heat conduits in a host of devices and materials. The strong in-plane graphitic carbon - carbon bonds make them exceptionally strong and stiff against axial strains. The almost zero in-plane thermal expansion but large inter-plane expansion of single walled nanotubes implies strong in-plane coupling and high flexibility against non-axial strains.
Many applications of CNTs, such as in nanoscale molecular electronics, sensing and actuating devices, or as reinforcing additive fibers in functional composite materials, have been proposed. Reports of several recent experiments on the preparation and mechanical characterization of CNT-polymer composites have also appeared. These measurements suggest modest enhancements in strength characteristics of CNT-embedded matrixes as compared to bare polymer matrixes. Preliminary experiments and simulation studies on the thermal properties of CNTs show very high thermal conductivity. It is expected, therefore, that nanotube reinforcements in polymeric materials may also significantly improve the thermal and thermomechanical properties of the composites.
Absorbtion
The large surface area and high absorbency of CNTs make them ideal candidates for use in air, gas, and water filtration. A lot of research is being done in replacing activated charcoal with CNTs in certain ultra high purity applications.
Uses
The special nature of carbon combined with the molecular perfection of single-walled nanotubes to endow them with exceptional material properties, such as very high electrical and thermal conductivity, strength, stiffness, and toughness. No other element in the periodic table bonds to itself in an extended network with the strength of the carbon-carbon bond. The delocalized pi-electron donated by each atom is free to move about the entire structure, rather than remain with its donor atom, giving rise to the first known molecule with metallic-type electrical conductivity. Furthermore, the high-frequency carbon-carbon bonds vibrations provide an intrinsic thermal conductivity higher than even diamond. In most conventional materials, however, the actual observed material properties - strength, electrical conductivity, etc. - are degraded very substantially by the occurrence of defects in their structure. For example, high-strength steel typically fails at only about 1% of its theoretical breaking strength. CNTs, however, achieve values very close to their theoretical limits because of their molecular perfection of structure.
Field Emission
CNTs are the best known field emitters of any material. This is understandable, given their high electrical conductivity, and the incredible sharpness of their tip. The smaller the tip’s radius of curvature, the more concentrated the electric field will be, leading to increased field emission. The sharpness of the tip also means that they emit at especially low voltage, an important fact for building low-power electrical devices that utilize this feature. CNTs can carry an astonishingly high current density. Furthermore, the current is extremely stable. An immediate application of this behavior receiving considerable interest is in field-emission flat-panel displays. Instead of a single electron gun, as in a traditional cathode ray tube display, in CNT-based displays there is a separate nanotube electron gun for each individual pixel in the display. Their high current density, low turn-on and operating voltages, and steady, long-lived behavior make CNTs very attractive field emitters in this application. Other applications utilizing the field-emission characteristics of CNTs include general types of low-voltage cold-cathode lighting sources, lightning arrestors, and electron microscope sources.
Conductive or Reinforced Plastics
Much of the history of plastics over the last half-century has involved their use as a replacement for metals. For structural applications, plastics have made tremendous headway, but not where electrical conductivity is required, because plastics are very good electrical insulators. This deficiency is overcome by loading plastics up with conductive fillers, such as carbon black and larger graphite fibers. The loading required to provide the necessary conductivity using conventional fillers is typically high, however, resulting in heavy parts, and more importantly, plastic parts whose structural properties are highly degraded. It is well-established that the higher the aspect ratio of the filler particles, the lower the loading required to achieve a given level of conductivity.
CNTs are ideal in this sense, since they have the highest aspect ratio of any carbon fiber. In addition, their natural tendency to form ropes provides inherently very long conductive pathways even at ultra-low loadings. Applications that exploit this behavior of CNTs include EMI/RFI shielding composites; coatings for enclosures, gaskets, and other uses such as electrostatic dissipation; antistatic materials, transparent conductive coatings; and radar-absorbing materials for stealth applications.
CNTs have the intrinsic characteristics desired in material used as electrodes in batteries and capacitors, two technologies of rapidly increasing importance. CNTs have a tremendously high surface area, good electrical conductivity, and very importantly, their linear geometry makes their surface highly accessible to the electrolyte.
Research has shown that CNTs have the highest reversible capacity of any carbon material for use in lithium ion batteries. In addition, CNTs are outstanding materials for super capacitor electrodes and are now being marketed for this application. CNTs also have applications in a variety of fuel cell components. They have a number of properties, including high surface area and thermal conductivity, which make them useful as electrode catalyst supports in PEM fuel cells. Because of their high electrical conductivity, they may also be used in gas diffusion layers, as well as current collectors. CNTs' high strength and toughness-to-weight characteristics may also prove valuable as part of composite components in fuel cells that are deployed in transport applications, where durability is extremely important.
Conductive Adhesives and Connectors
The same properties that make CNTs attractive as conductive fillers for use in electromagnetic shielding, ESD materials, etc., make them attractive for electronics packaging and interconnection applications, such as adhesives, potting compounds, coaxial cables, and other types of connectors.
Molecular Electronics
The idea of building electronic circuits out of the essential building blocks of materials - molecules - has seen a revival the past few years, and is a key component of nanotechnology. In any electronic circuit, but particularly as dimensions shrink to the nanoscale, the interconnections between switches and other active devices become increasingly important. Their geometry, electrical conductivity, and ability to be precisely derived, make CNTs the ideal candidates for the connections in molecular electronics. In addition, they have been demonstrated as switches themselves.
There are already companies such as Nantero from Woburn, MA that are already making CNT based non-volitle random access memory for PC’s. A lot of research is being done to design CNT based transistors as well.
Thermal Materials
The record-setting anisotropic thermal conductivity of CNTs is enabling many applications where heat needs to move from one place to another. Such an application is found in electronics, particularly heat sinks for chips used in advanced computing, where uncooled chips now routinely reach over 100oC. The technology for creating aligned structures and ribbons of CNTs [D.Walters, et al., Chem. Phys. Lett. 338, 14 (2001)] is a step toward realizing incredibly efficient heat conduits. In addition, composites with CNTs have been shown to dramatically increase their bulk thermal conductivity, even at very small loadings.
The superior properties of CNTs are not limited to electrical and thermal conductivities, but also include mechanical properties, such as stiffness, toughness, and strength. These properties lead to a wealth of applications exploiting them, including advanced composites requiring high values of one or more of these properties.
Fibers and Fabrics
Fibers spun of pure CNTs have recently been demonstrated and are undergoing rapid development, along with CNT composite fibers. Such super-strong fibers will have many applications including body and vehicle armor, transmission line cables, woven fabrics and textiles.
Catalyst Support
CNTs intrinsically have an enormously high surface area; in fact, for single walled nanotubes every atom is not just on one surface - each atom is on two surfaces, the inside and the outside of the nanotube! Combined with the ability to attach essentially any chemical species to their sidewalls this provides an opportunity for unique catalyst supports. Their electrical conductivity may also be exploited in the search for new catalysts and catalytic behavior.
CNT Ceramics
A ceramic material reinforced with carbon nanotubes has been made by materials scientists at UC Davis. The new material is far tougher than conventional ceramics, conducts electricity and can both conduct heat and act as a thermal barrier, depending on the orientation of the nanotubes. Ceramic materials are very hard and resistant to heat and chemical attack, making them useful for applications such as coating turbine blades, but they are also very brittle.
The researchers mixed powdered alumina (aluminum oxide) with 5 to 10 percent carbon nanotubes and a further 5 percent finely milled niobium. The researchers treated the mixture with an electrical pulse in a process called spark-plasma sintering. This process consolidates ceramic powders more quickly and at lower temperatures than conventional processes.
The new material has up to five times the fracture toughness -- resistance to cracking under stress -- of conventional alumina. The material shows electrical conductivity seven times that of previous ceramics made with nanotubes. It also has interesting thermal properties, conducting heat in one direction, along the alignment of the nanotubes, but reflecting heat at right angles to the nanotubes, making it an attractive material for thermal barrier coatings.
Biomedical Applications
The exploration of CNTs in biomedical applications is just underway, but has significant potential. Since a large part of the human body consists of carbon, it is generally thought of as a very biocompatible material. Cells have been shown to grow on CNTs, so they appear to have no toxic effect. The cells also do not adhere to the CNTs, potentially giving rise to applications such as coatings for prosthetics and surgical implants. The ability to functionalize the sidewalls of CNTs also leads to biomedical applications such as vascular stents, and neuron growth and regeneration. It has also been shown that a single strand of DNA can be bonded to a nanotube, which can then be successfully inserted into a cell; this has potential applications in gene therapy.
Air, Water and Gas Filtration
Many researchers and corporations have already developed CNT based air and water filtration devices. It has been reported that these filters can not only block the smallest particles but also kill most bacteria. This is another area where CNTs have already been commercialized and products are on the market now. Someday CNTs may be used to filter other liquids such as fuels and lubricants as well.
A lot of research is being done in the development of CNT based air and gas filtration. Filtration has been shown to be another area where it is cost effective to use CNTs already. The research I’ve seen suggests that 1 gram of MWNTs can be dispersed onto 1 sq ft of filter media. Manufacturers can get their cost down to 35 cents per gram of purified MWNTs when purchasing ton quantities.
Other Applications
Some commercial products on the market today utilizing CNTs include stain resistant textiles, CNT reinforced tennis rackets and baseball bats. Companies like Kraft foods are heavily funding cnt based plastic packaging. Food will stay fresh longer if the packaging is less permeable to atmosphere. Coors Brewing company has developed new plastic beer bottles that stay cold for longer periods of time. Samsung already has CNT based flat panel displays on the market. A lot of companies are looking forward to being able to produce transparent conductive coatings and phase out ITO coatings. Samsung uses align SWNTs in the transparent conductive layer of their display manufacturing process.
Source : nanowerk.com




