Fullerenes are a form of carbon molecule that is neither graphite nor diamond. They consist of a spherical, ellipsoid, or cylindrical arrangement of dozens of carbon atoms. Fullerenes were named after Richard Buckminster Fuller, an architect known for the design of geodesic domes which resemble spherical fullerenes in appearance. A spherical fullerene looks like a soccer ball, and are often called "buckyballs," whereas cylindrical fullerenes are known as "buckytubes" or "nanotubes."
Fullerenes were discovered as an unexpected surprise during laser spectroscopy experiments at Rice University in September 1985. The 1996 Nobel Prize in Chemistry was awarded to Professors Robert F. Curl, Jr., Richard E. Smalley, and Sir Harold W. Kroto for their discovery. Fullerene molecules consist of 60, 70, or more carbon atoms, unlike diamond and graphite, the more familiar forms of carbon.
Fullerenes occur only in small amounts naturally, but several techniques for producing them in greater volumes have been suggested. The modern technique uses a benzene flame to produce fullerenes. Other techniques include the vaporization of graphite rods and catalytic chemical vapor deposition from ethanol vapor.
The fullerene family of carbon molecules possess a range of unique properties. A fullerene nanotube has tensile strength about 20 times that of high-strength steel alloys, and a density half that of aluminum. Carbon nanotubes demonstrate superconductive properties, and single nanotubes up to 4 centimeters in length have been synthesized. A range of companies exists to develop nanotubes for commercial applications, including computer memory, electronic wires, and materials science. One day nanotubes could be used to create futuristic computers not possible with conventional lithographic techniques.
Nanotubes have been a central focus in the buzz surrounding the emerging field of "nanotechnology." The association is sometimes misleading; when physicist Richard Feynman originally proposed building manufacturing systems that assemble products on the molecular level ("molecular nanotechnology"), he was talking about tiny, productive machine systems, not the creation of exotic nano-scale materials like fullerenes using macro-scale chemistry techniques. A tiny factory built entirely out of fullerenes would qualify as molecular nanotechnology, but fullerenes on their own do not. This is a critical distinction often overlooked by some academics, venture capitalists, and technologists who are fond of using the word "nanotechnology" as a tool to attract funding or attention.
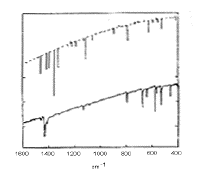
The infra red spectrum of C60(left); 13C NMR spectrum of C60 (right):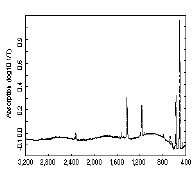
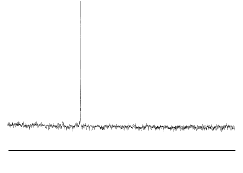
C60 only has four IR active vibrational bands, due to its icosohedral symmetry.
The UV/visual spectrum chromatographically purified C60 (left); A mass spectrum showing the higher fullerenes present in carbon soot (right):
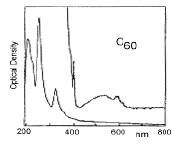
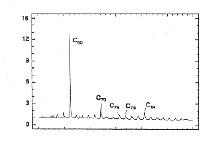
Mass spectrometry has been widely used to study the fullerenes. There is evidence for species as small as C20+, as well as stable peaks for the cluster ions C2n+ (where 2n>32).
Physical properties of C60:
Density: 1.65 g cm-3
Standard heat of formation: 9.08 k cal mol-1
Index of refraction: 2.2 (600nm)
Boiling point: Sublimes at 800K
Resistivity: 1014 ohms m-1
Vapour density: N/A
Crystal form Hexagonal cubic
Vapour pressure: 5 x 10-6 torr at room temperature
8 x 10-4 torr at 800K
Organoleptic properties:
Appearance
Buckyball soot: Very finely divided black powder
Fullerite: Brown/black powder
C60: Black solid
Odour: Odourless
The fullerenes are also found to be soluble in common solvents such as benzene, toluene or chloroform. If one shakes up some fullerene soot with toluene and filter the mixture, one obtains a red solution. As the solvent evaporates, crystals of pure carbon appear
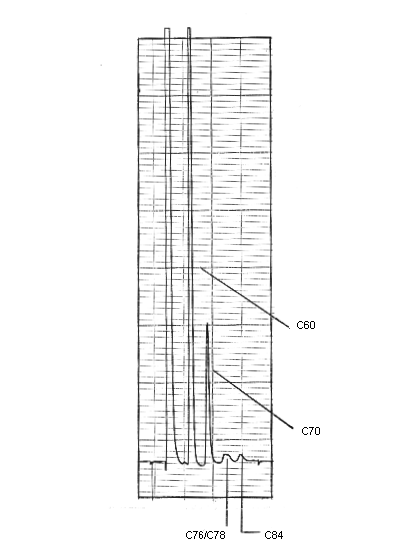
An HPLC trace showing fullerenes found in carbon soot:
C70
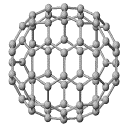 Structure of C70
Structure of C70
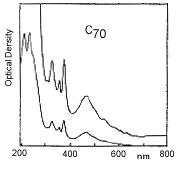
Infra red spectrum of C70 (left); UV/visual spectrum of C70 (right):
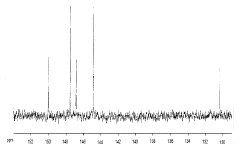
Carbon 13 NMR spectrum of C70:
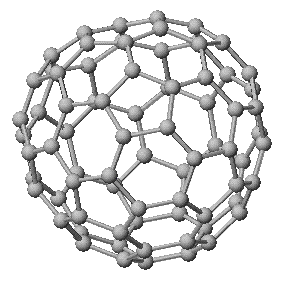
C76
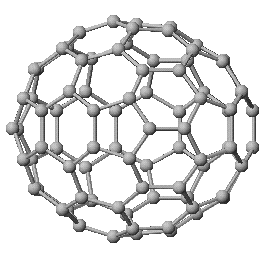
C84
"Buckytubes" & "Buckyonions"
Many fullerenes have now been discovered in carbon soot, uncovered by electron microscopy, including tubes of carbon many thousands of times long as they are wide, with the same icosahedral structure as the fullerenes. These have diameters as small as 2nm. Carbon "onions" have also been discovered, and consist of carbon cages one inside the other rather like Russian dolls. These carbon particles have millions of atoms, and many have been observed with dozens of concentric shells. Hypothetical structures have been postulated for carbon cages consisting of not pentagonal and hexagonal rings (like C60), but heptagonal (7 membered) rings.
The reactions and reaction types of the fullerenes described below are merely examples of some recent work, not an exhaustive study.
General
Redox chemistry
C60 and C70 have similar properties, with six reversible one electron reductions to C60(6-) and C70(6-) having been observed. Oxidation of C60 and C70 is, however irreversible. The first reduction for both fullerenes is ~1.0V for (Fc/Fc+), indicating they have electron accepting properties. C76 exhibits both electron donor/acceptor properties.
C60 is an "alkene"
C60 has a tendency of avoiding having double bonds within the pentagonal rings which makes electron delocalisation poor, and results in the fact that C60 is not "superaromatic". C60 behaves very much like an electron deficient alkene and readily reacts with electron rich species (see its organometallic reactions), but can also sometimes behave in a similar way to aromatic rings.
Classes of fullerene compunds
Compounds of fullerenes may be classed according to two different catagories: Exohedral (inside the cage) and Endohedral (outside the cage). Examples of the former include metals such as La enclosed in a C82 cage, and examples of the latter include transition metal complexes eg with Ir, as well as purely organic fragments bonded to the fullerene cage.
Organic Fullerene Chemistry
Fully brominated C60: C60Br(24)
As noted above, fullerenes behave as electron deficient alkenes, and will react with electron rich species, such as halogens. Formation of C60Br24 by reactio of solid C60 with neat liquid bromine takes 5-8 days, but recently (Sept. 1996) a new method has been developed at Widener University, which reduces the reaction time to about an hour.
Experimental : C60 (50mg) is placed in a closed conatiner with neat liquid bromine (5ml) and large gauge iron wire (ca. 1g), and stirred at room temp. for 1h. The excess liq. Br is then removed by vacuum evaporation, and the resulting solid treated with methanol to remove FeBr3 formed during the reaction. The remaining solid is then filtered and washed with methanol.
When C60 was first discovered, new experiments were rapidly devised to test the C60 (as a closed cage structure) hypothesis. As the C60 structure is hollow, with room for one or more other atoms, attempts were made to enclose a metal atom. A graphite sheet was soaked with a solution of a metal salt (lanthanum chloride, LaCl3) and subjected to vaporisation-condensation
experiments. Mass spectroscopic analysis of the clusters formed showed the presence of C60La+. These proved to be photoresistant, reinforcing the idea that metal atoms were "captured" inside the cage structure. The so called "shrink wrapping" experiment was then devised whereby ions of one size (or similar size) were captured in a magnetic trap and subjected to a laser pulse. The laser pulse causes the carbon cage to shrink by 2 carbon atoms at a time, until fragmentation ceased when pressure on the trapped metal atom is too big. The carbon cage now fits exactly around the metal atom. For example, (forC60Cs+) this size is at (C48Cs+) and for (C60K+) it is at (C44K+).
So Y dont a Vulcanised tyre.....?
Fullerenes were discovered as an unexpected surprise during laser spectroscopy experiments at Rice University in September 1985. The 1996 Nobel Prize in Chemistry was awarded to Professors Robert F. Curl, Jr., Richard E. Smalley, and Sir Harold W. Kroto for their discovery. Fullerene molecules consist of 60, 70, or more carbon atoms, unlike diamond and graphite, the more familiar forms of carbon.
Fullerenes occur only in small amounts naturally, but several techniques for producing them in greater volumes have been suggested. The modern technique uses a benzene flame to produce fullerenes. Other techniques include the vaporization of graphite rods and catalytic chemical vapor deposition from ethanol vapor.
The fullerene family of carbon molecules possess a range of unique properties. A fullerene nanotube has tensile strength about 20 times that of high-strength steel alloys, and a density half that of aluminum. Carbon nanotubes demonstrate superconductive properties, and single nanotubes up to 4 centimeters in length have been synthesized. A range of companies exists to develop nanotubes for commercial applications, including computer memory, electronic wires, and materials science. One day nanotubes could be used to create futuristic computers not possible with conventional lithographic techniques.
Nanotubes have been a central focus in the buzz surrounding the emerging field of "nanotechnology." The association is sometimes misleading; when physicist Richard Feynman originally proposed building manufacturing systems that assemble products on the molecular level ("molecular nanotechnology"), he was talking about tiny, productive machine systems, not the creation of exotic nano-scale materials like fullerenes using macro-scale chemistry techniques. A tiny factory built entirely out of fullerenes would qualify as molecular nanotechnology, but fullerenes on their own do not. This is a critical distinction often overlooked by some academics, venture capitalists, and technologists who are fond of using the word "nanotechnology" as a tool to attract funding or attention.
The infra red spectrum of C60(left); 13C NMR spectrum of C60 (right):


C60 only has four IR active vibrational bands, due to its icosohedral symmetry.
The UV/visual spectrum chromatographically purified C60 (left); A mass spectrum showing the higher fullerenes present in carbon soot (right):


Mass spectrometry has been widely used to study the fullerenes. There is evidence for species as small as C20+, as well as stable peaks for the cluster ions C2n+ (where 2n>32).
Physical properties of C60:
Density: 1.65 g cm-3
Standard heat of formation: 9.08 k cal mol-1
Index of refraction: 2.2 (600nm)
Boiling point: Sublimes at 800K
Resistivity: 1014 ohms m-1
Vapour density: N/A
Crystal form Hexagonal cubic
Vapour pressure: 5 x 10-6 torr at room temperature
8 x 10-4 torr at 800K
Organoleptic properties:
Appearance
Buckyball soot: Very finely divided black powder
Fullerite: Brown/black powder
C60: Black solid
Odour: Odourless
The fullerenes are also found to be soluble in common solvents such as benzene, toluene or chloroform. If one shakes up some fullerene soot with toluene and filter the mixture, one obtains a red solution. As the solvent evaporates, crystals of pure carbon appear
An HPLC trace showing fullerenes found in carbon soot:
C70
 Structure of C70
Structure of C70Infra red spectrum of C70 (left); UV/visual spectrum of C70 (right):


Carbon 13 NMR spectrum of C70:

C76
C84
"Buckytubes" & "Buckyonions"
Many fullerenes have now been discovered in carbon soot, uncovered by electron microscopy, including tubes of carbon many thousands of times long as they are wide, with the same icosahedral structure as the fullerenes. These have diameters as small as 2nm. Carbon "onions" have also been discovered, and consist of carbon cages one inside the other rather like Russian dolls. These carbon particles have millions of atoms, and many have been observed with dozens of concentric shells. Hypothetical structures have been postulated for carbon cages consisting of not pentagonal and hexagonal rings (like C60), but heptagonal (7 membered) rings.
The reactions and reaction types of the fullerenes described below are merely examples of some recent work, not an exhaustive study.
General
Redox chemistry
C60 and C70 have similar properties, with six reversible one electron reductions to C60(6-) and C70(6-) having been observed. Oxidation of C60 and C70 is, however irreversible. The first reduction for both fullerenes is ~1.0V for (Fc/Fc+), indicating they have electron accepting properties. C76 exhibits both electron donor/acceptor properties.
C60 is an "alkene"
C60 has a tendency of avoiding having double bonds within the pentagonal rings which makes electron delocalisation poor, and results in the fact that C60 is not "superaromatic". C60 behaves very much like an electron deficient alkene and readily reacts with electron rich species (see its organometallic reactions), but can also sometimes behave in a similar way to aromatic rings.
Classes of fullerene compunds
Compounds of fullerenes may be classed according to two different catagories: Exohedral (inside the cage) and Endohedral (outside the cage). Examples of the former include metals such as La enclosed in a C82 cage, and examples of the latter include transition metal complexes eg with Ir, as well as purely organic fragments bonded to the fullerene cage.
Organic Fullerene Chemistry
Fully brominated C60: C60Br(24)
As noted above, fullerenes behave as electron deficient alkenes, and will react with electron rich species, such as halogens. Formation of C60Br24 by reactio of solid C60 with neat liquid bromine takes 5-8 days, but recently (Sept. 1996) a new method has been developed at Widener University, which reduces the reaction time to about an hour.
Experimental : C60 (50mg) is placed in a closed conatiner with neat liquid bromine (5ml) and large gauge iron wire (ca. 1g), and stirred at room temp. for 1h. The excess liq. Br is then removed by vacuum evaporation, and the resulting solid treated with methanol to remove FeBr3 formed during the reaction. The remaining solid is then filtered and washed with methanol.
When C60 was first discovered, new experiments were rapidly devised to test the C60 (as a closed cage structure) hypothesis. As the C60 structure is hollow, with room for one or more other atoms, attempts were made to enclose a metal atom. A graphite sheet was soaked with a solution of a metal salt (lanthanum chloride, LaCl3) and subjected to vaporisation-condensation
experiments. Mass spectroscopic analysis of the clusters formed showed the presence of C60La+. These proved to be photoresistant, reinforcing the idea that metal atoms were "captured" inside the cage structure. The so called "shrink wrapping" experiment was then devised whereby ions of one size (or similar size) were captured in a magnetic trap and subjected to a laser pulse. The laser pulse causes the carbon cage to shrink by 2 carbon atoms at a time, until fragmentation ceased when pressure on the trapped metal atom is too big. The carbon cage now fits exactly around the metal atom. For example, (forC60Cs+) this size is at (C48Cs+) and for (C60K+) it is at (C44K+).
So Y dont a Vulcanised tyre.....?




