Leather tanning is the process of converting raw hides or skins into leather. Hides and skins have the
Leather tanning is the process of converting raw hides or skins into leather. Hides and skins have theability to absorb tannic acid and other chemical substances that prevent them from decaying, make them resistant to wetting, and keep them supple and durable. The surface of hides and skins contains the hair and oil glands and is known as the grain side. The flesh side of the hide or skin is much thicker and softer. The three types of hides and skins most often used in leather manufacture are from cattle, sheep, and pigs.
Tanning is essentially the reaction of collagen fibers in the hide with tannins, chromium, alum, or
other chemical agents. The most common tanning agents used in the U. S. are trivalent chromium and
vegetable tannins extracted from specific tree barks. Alum, syntans (man-made chemicals), formaldehyde,
glutaraldehyde, and heavy oils are other tanning agents.
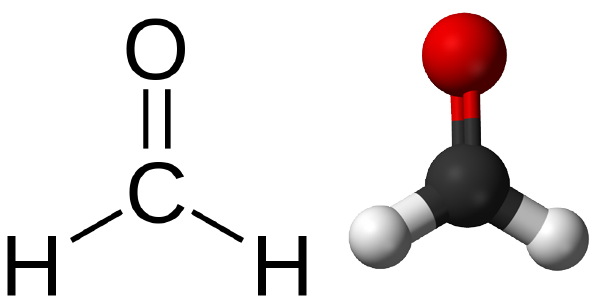
Formaldehyde (a pungent smelling gas) is water soluble and its solution is known as formalin(toxic and may develop acidity). Presumably tanning was observed for the first time when meat together with the skin were smoked. Formaldehyde is probably the only tanning gas. Stabilized formalin(containing 8-10 % MeOH) contains 40 % formaldehyde and is used for tanning white, washable leathers with the grain split or shaved off.
The skins are prepared to a pH 4 or 5 and drummed in 3% formalin with the least possible amount of water. A temperature of 30 C is beneficial. After runtime of 4-5 hours, they are left in the closed drum overnight and then 'ashed up' (1-1.5 % soda ash, 50-100% water) until pH is not less than 8. At this pH formaldehyde rapidly fixes to the skins. At higher pHs over tannage of grain side (with no penetration) occurs. If production of grain leather is intended various(modified) alkali systems are recomended (use of Mg salts) to avoid this danger.
The amount of aldehyde being attached to the hide is small, from 0.2 to 2 %. Part of this may remain unbound. Aldehydes combine with the basic amino group of skin protein. In alkali some condensation(aldol) to larger molecules give fullness to the leather.
Aldehyde tanned leathers have reduced ability to fix acid (basic groups have reacted).Similarly they can reduce fixation of some vegetable tans and dyes. Aldehyde tannage reduces isoelectric point of hides, so that at any pH it has a lower cationic charge than raw skin, and mineral tanned leather. This can reduce fixation of anionic sulphated oils so that such fatliquors penetrate better, but may washout more easily.
Ts is raised only to 70 C. Leather becomes whiter as exposed to light and readily absorbs water.
RNH3+ + CH2O --> R-NH-CH2OH + H+
RNH-CH2OH + NH2CO-R --> RNH-CH2 - NH-CO-R + H2O Gluteraldehyde: (OCH-CH2CH2-CHO)
Aldehyde tanned leathers have reduced ability to fix acid (basic groups have reacted).Similarly they can reduce fixation of some vegetable tans and dyes. Aldehyde tannage reduces isoelectric point of hides, so that at any pH it has a lower cationic charge than raw skin, and mineral tanned leather. This can reduce fixation of anionic sulphated oils so that such fatliquors penetrate better, but may washout more easily.
Ts is raised only to 70 C. Leather becomes whiter as exposed to light and readily absorbs water.
RNH3+ + CH2O --> R-NH-CH2OH + H+
RNH-CH2OH + NH2CO-R --> RNH-CH2 - NH-CO-R + H2O Gluteraldehyde: (OCH-CH2CH2-CHO)
Under equivalent conditions it can give higher degree of tannage and increase of Ts than formaldehyde at lower pHs. Attention has been given to the phenomena that some degree of gluteraldehyde particularly on mineral tannages improves leathers resistance to perspiration.
Gluteraldehyde forms semiacetal bonds with hydroxyls of hydroxyproline, hydroxylysine and serine. With phenols it yields insoluble compounds, so can not be used with vegetable tannins.
Gluteraldehyde forms semiacetal bonds with hydroxyls of hydroxyproline, hydroxylysine and serine. With phenols it yields insoluble compounds, so can not be used with vegetable tannins.

With amino groups it my react in 3 ways:

Gluteraldehyde in 25-50% aqueous solution is found to oligomerize (3-5 molecules).This may be prevented by addition of alcohol(at low temperature)
The Advantages of these Synthetic Leather
Synthetic leather is manufactured in rolls. The thickness is exact. It contains engineered polymers that are chemically linked to give the strength of leather without the brittleness. Ends made of Synthetic leather are very easy to get on over a guitar strap button. We have tested the synthetic leather ends extensively and have received only positive comments from our testers. The synthetic leather has the same embossed graining as the finished split leather ends so it looks just like the "genuine" leather. The synthetic leather ends are easier to work with and hold up as well if not better than cowhide leather when used for guitar straps ends. Synthetic leather has all of the best elements of cowhide without all the bad characteristics of cowhide. Due to superiority of the synthetic leather material ALL Legacy Guitar straps will now come standard with Synthetic leather ends. Since no animals are harmed to produce these products.Courtesy :chem.boun.edu.tr